Rare Disease Copy Number Analysis
What is our Copy Number Analysis test?
This test detects genome-wide copy number variants correlated to a patient’s symptoms or phenotype.


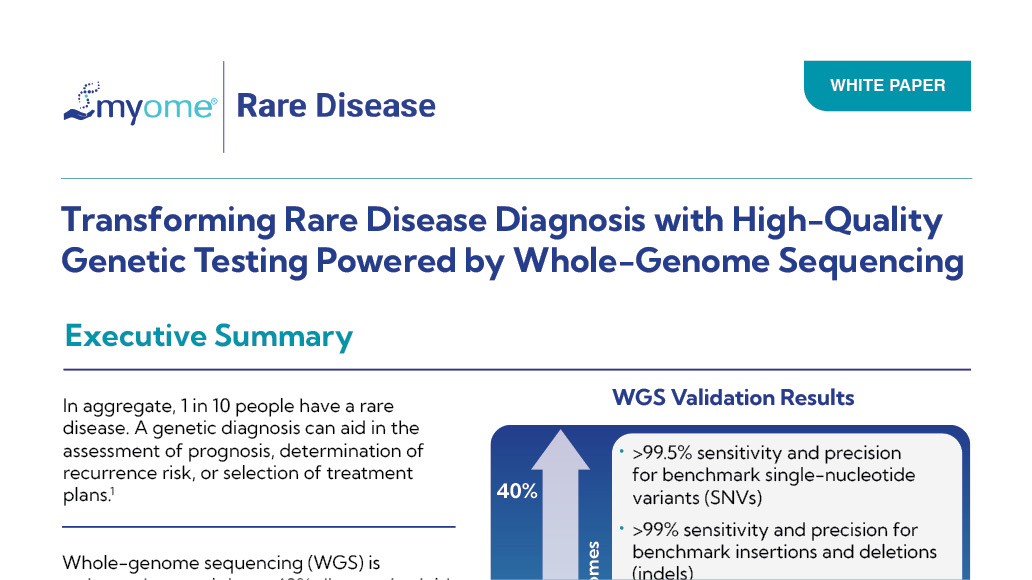
Whole Genome Sequencing for CNV Detection
This test uses whole genome sequencing to identify genome-wide copy number variations, deletions and duplications, related to the patient's phenotype.


Copy Number Analysis may be considered for patients with the following:
- Multiple congenital anomalies (MCA)1
- Developmental delay (DD)1
- Intellectual disability (ID)1
- Autism spectrum disorder (ASD)1
- Miller, David T., et al. "Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies." The American Journal of Human Genetics 86.5 (2010): 749-764.
Test Code
PR51001
Turn Around Times
From initial sample received, approximately 5 to 6 weeks*
Acceptable Sample Types
- Blood
- Buccal
- Saliva
Acceptable Billing Types
- Insurance Coverage
- Institutional
- Self-Pay
- Financial Assistance (such as interest-free payment plans, income and family based financial assistance, and prompt-pay discounts if available)
CPT Codes
81349**
Included
- CNV report similar to a CMA report
- Comprehensive report with known actionable recommendations and relevant variants of uncertain significance
- Confirmation of reported variants by a secondary technology if warranted
Optional
- Reflex to Exome or Genome Analysis
- Fragile X testing
- Genetic counseling
Interpreting Rare Disease Copy Number Analysis results


Order a Rare Disease Copy Number test
We make it easy to order MyOme's Rare Disease Copy Number test. Talk to your healthcare provider to learn more and start diagnostic testing today.